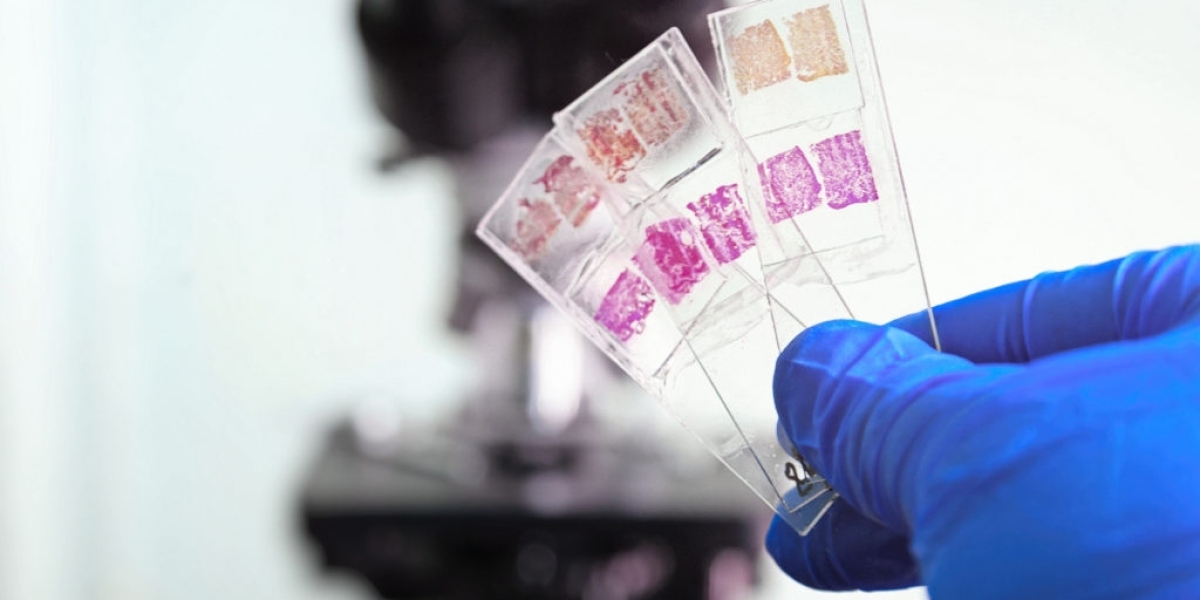
Immunohistochemistry (IHC) refers to the process of detecting antigens (ie, proteins) in cells of a tissue section by exploiting the principles of antibodies binding specifically to antigens in biological tissues. In IHC, the detection of antigen-antibody binding reactions is accomplished by tagging antibodies with enzymatic/fluorescent/radioactive labels. When substrates react with these labels attached to antibodies, a visible reaction product is formed allowing the antigens to be identified and visualized under a microscope. IHC sees wide utility as a diagnostic tool in pathology laboratories and as a method for cell identification and cell localization in biomedicine and life science research.
Basic Principles of IHC
The basic steps involved in IHC are: tissue/cell fixation, antigen retrieval, blocking, primary antibody incubation, washing, secondary antibody incubation, detection, and visualization. Tissue/cell specimens are first fixed in formalin or frozen to preserve the antigenicity of molecules. Antigen retrieval techniques are utilized to unmask antigens that may be obscured during fixation. Endogenous enzymatic activity and nonspecific binding sites are blocked with carrier proteins. Next, the sample is incubated with the primary antibody against the target antigen. After washing away unbound primary antibodies, the sample is incubated with a secondary antibody carrying an enzyme or fluorophore label. Upon addition of a chromogenic/fluorogenic substrate, the reaction product forms at the antigen sites, allowing visualization under a microscope.
Applications of IHC
IHC has widespread applications in clinical diagnostics, research, and more. In diagnostic pathology, IHC helps characterize tumors, determine the lineage/type of hematological malignancies, and diagnose infectious diseases. Pharmaceutical/biotech industries utilize IHC to quantify target expression levels during drug development. In basic research, IHC localization of specific proteins provides insights into developmental biology, organogenesis, gene function, and disease mechanisms. IHC also finds use in neuroscience to map neural circuits, in stem cell biology to track cell lineage, and in cancer biology to study oncogenesis, metastasis, and tumor microenvironments. The ability of IHC to identify multiple antigens simultaneously on a single slide has enhanced its role in accurate diagnostics and comprehensive biomarker profiling.
Types of Labels Used in IHC
The most commonly used labels in IHC are:
- Enzyme labels - Horseradish peroxidase (HRP) and alkaline phosphatase are the enzymes most frequently conjugated to secondary antibodies in IHC. Their reaction with chromogenic substrates yields a visible colored precipitate.
- Fluorescent labels - Fluorophores like FITC, TRITC, Cyanine dyes are excited by light of a specific wavelength and emit light of longer wavelengths, allowing fluorescence microscopy detection. Fluorescent IHC offers multiplexing capabilities.
- Radioactive labels - Isotopes like 125I, 3H, 14C incorporated into antibodies enable radioactive IHC detection using autoradiography or liquid scintillation counting. However, radioactivity poses hazards limiting its routine use.
- Heavy metal labels - Gold, silver, and other metals conjugated to secondary antibodies can be detected by transmitted light or electron microscopy after precipitation reactions. Although not widely used now, they were groundbreaking in early IHC development.
- Enzyme-cycling reporter system - Highly sensitive detection systems like Tyramide Signal Amplification utilize HRP to deposit numerous horseradish peroxidase molecules in close proximity to primary antibody binding sites, amplifying the detection signal.
Automation and Digital Analysis in IHC
IHC saw technological advancements with the introduction of automated slide staining systems that standardize protocols, maximize throughput and minimize hands-on time. Digital whole slide imaging coupled with sophisticated image analysis software has enabled quantitative IHC. Computer-aided scoring and algorithms can quantify biomarker expression levels, characterizing staining intensity, distribution and subcellular localization. When combined with clinical outcomes, associations between prognostic/predictive biomarkers and patient survival/response to therapy can be determined. These quantitative techniques have enhanced the role of IHC in precision medicine applications.
Limitations and Challenges of IHC
While IHC is a widely employed technique, there are inherent limitations. Antigenicity may be lost during fixation/processing necessitating retrieval methods. Non-specific binding can produce background staining. Limited penetration of antibodies into thick tissue sections poses challenges. Quantification is semi-quantitative rather than absolute. Multiparametric analysis requires optimization of multiple markers on the same slide. Long-term antigen stability poses pre-analytic issues. Inter-observer variability in morphologic assessment is a concern. Despite technological improvements, complete automation is still elusive. Standardization of protocols and validation of new markers remain challenges. Addressing these limitations through methodological innovations will further advance IHC applications.
In summary, immunohistochemistry is a versatile and powerful technique that has become an indispensable tool in histopathology, diagnostics and biomedical research. Continuous methodological refinements and automation/digital advancements are expanding the applications and capabilities of IHC for precision medicine solutions.
Costa Roger
39 Blog posts