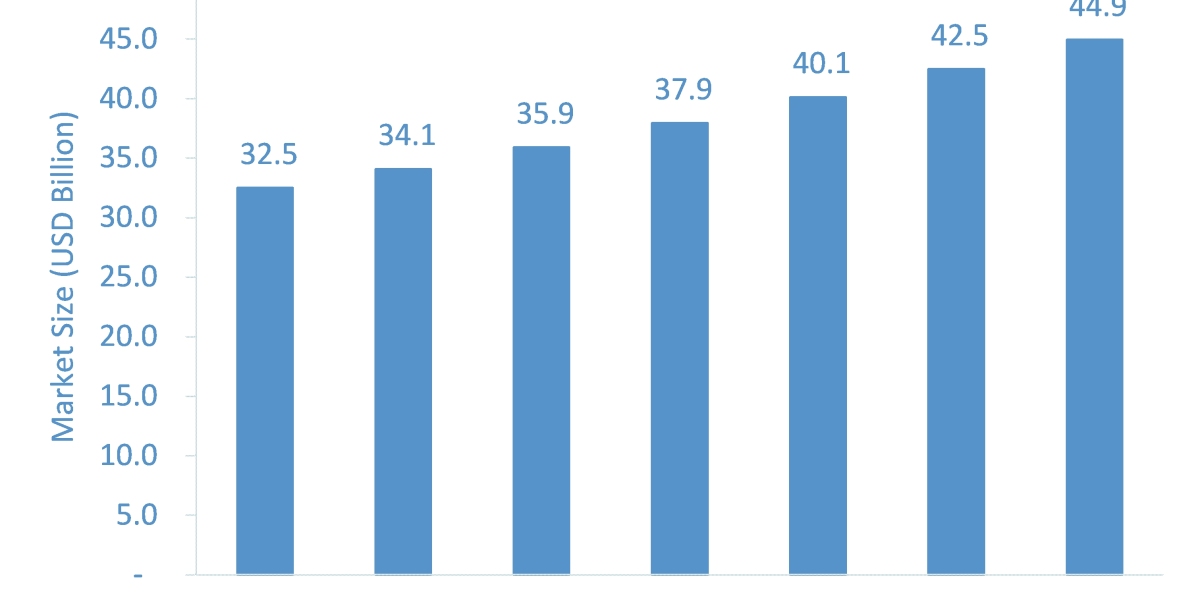
Brief Overview of Ivermectin:
- Originally developed as an antiparasitic medication.
- Widely used to treat various parasitic infections in humans and animals.
- Recognized for its safety and efficacy, particularly in combating diseases caused by roundworms and parasites.
Emergence of Ivermectin in Discussions Related to COVID-19 Treatment:
- Initial Recognition: a. Ivermectin Where To Buy gained attention due to in vitro studies suggesting antiviral properties. b. Preliminary positive outcomes in observational studies fueled interest in its potential against SARS-CoV-2.
- Global Debate: a. Ivermectin became a subject of global debate as discussions regarding its use for COVID-19 treatment intensified. b. Advocates argue for its potential as a low-cost, widely available treatment option. c. Skepticism and caution exist due to the need for more robust clinical evidence.
- Media and Public Interest: a. Media coverage amplified public interest in Ivermectin as a possible COVID-19 treatment. b. Varied narratives and interpretations contribute to the complexity of public understanding.
Note: The emergence of Ivermectin in discussions surrounding COVID-19 treatment highlights the need for careful evaluation of scientific evidence and responsible communication to the public. Ongoing research aims to provide clarity on its effectiveness and safety in combating the virus.
What is Ivermectin?
Background and History of Ivermectin:
- Discovery: a. Developed by Japanese researcher Satoshi Ōmura and American scientist William C. Campbell. b. Isolated from Streptomyces avermitilis, a soil bacterium.
- Nobel Prize Recognition: a. Awarded the Nobel Prize in Physiology or Medicine in 2015 for its impact on global health. b. Significantly contributed to the control of diseases caused by parasitic worms.
Approved Uses and Effectiveness in Treating Parasitic Infections:
- Onchocerciasis (River Blindness): a. Approved for mass drug administration programs to control onchocerciasis in endemic regions. b. Highly effective in reducing the burden of Onchocerca volvulus.
- Lymphatic Filariasis: a. Used to control lymphatic filariasis, targeting Wuchereria bancrofti.
- Strongyloidiasis and Other Parasitic Infections: a. Effective against various nematode and arthropod parasites. b. Demonstrated efficacy in treating strongyloidiasis, scabies, and other parasitic conditions.
Mechanism of Action:
- Neurological Impact: a. Binds to glutamate-gated chloride channels in nerve and muscle cells of parasites. b. Causes an increase in membrane permeability to chloride ions, leading to hyperpolarization and paralysis of the parasite.
- Selectivity: a. The mechanism is more selective for invertebrate nerve and muscle cells, contributing to the safety profile in humans.
- Immune Modulation: a. Emerging evidence suggests potential immunomodulatory effects, contributing to its antiviral properties.