Market Overview:
Report Attribute | Key Statistics |
|---|---|
| Base Year | 2022 |
| Forecast Years | 2023-2033 |
| Historical Years | 2017-2022 |
Market Size in 2022 | US$ 466.1 Million |
Market Forecast in 2033 | US$ 1,786.1 Million |
Market Growth Rate (2023-2033) | 12.8% |
The dystrophic epidermolysis bullosa market reached a value of US$ 466.1 Million in 2022 and expects to reach US$ 1,786.1 Million by 2033, exhibiting a growth rate (CAGR) of 12.8% during 2023-2033.
The dystrophic epidermolysis bullosa market report offers a comprehensive analysis of the market in the United States, EU5 (including Germany, Spain, Italy, France, and the United Kingdom), and Japan. It covers aspects such as treatment methods, drugs available in the market, drugs in development, the market share of various therapies, and the market's performance in the seven major regions. Additionally, the report evaluates the performance of leading companies and their pharmaceutical products. Current and projected patient numbers across these key markets are also detailed in the report. This study is essential for manufacturers, investors, business planners, researchers, consultants, and anyone interested or involved in the dystrophic epidermolysis bullosa market.
Request for a Free Sample of this Report: https://www.imarcgroup.com/dystrophic-epidermolysis-bullosa-market/requestsample
Dystrophic Epidermolysis Bullosa Market Trends:
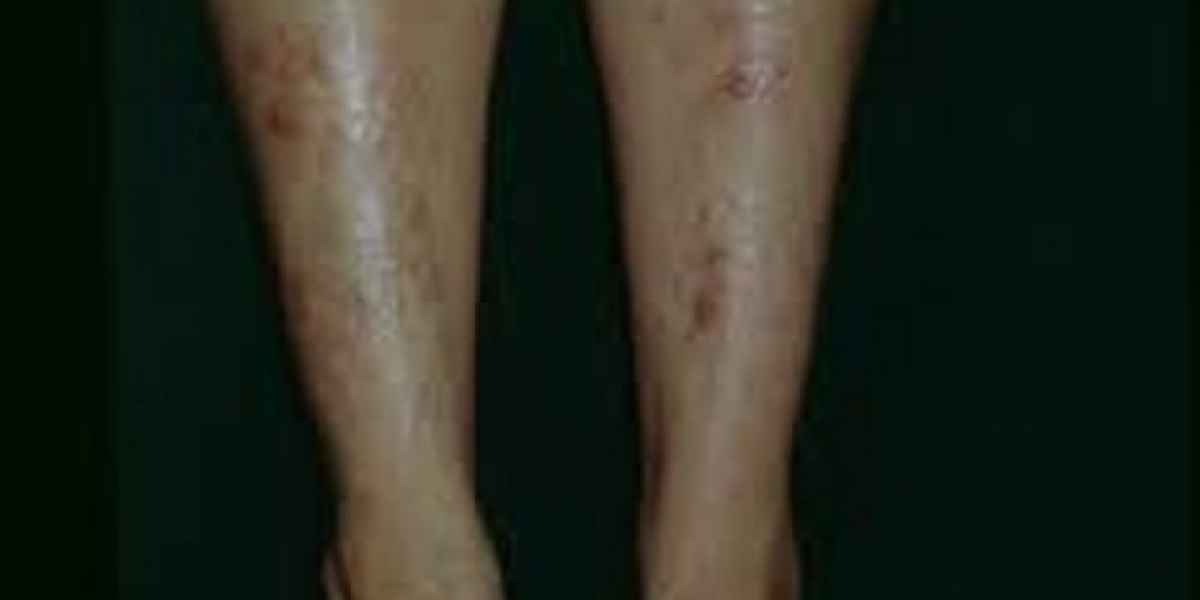
Dystrophic epidermolysis bullosa is a rare and devastating genetic skin disorder characterized by extreme fragility of the skin and mucous membranes. The market is witnessing significant advancements and growth, primarily driven by several key factors. Growing awareness among patients, caregivers, and healthcare professionals about dystrophic epidermolysis bullosa has led to improved early diagnosis. Early detection is crucial for managing the condition effectively, leading to a greater demand for treatment and care options. Regulatory agencies worldwide have granted orphan drug status to many dystrophic epidermolysis bullosa treatments. This designation provides incentives for pharmaceutical companies to develop treatments for rare diseases like dystrophic epidermolysis bullosa, driving research and development efforts. Also, the introduction of genetic therapies, such as gene editing and gene therapy, is a major driver in the dystrophic epidermolysis bullosa market. These cutting-edge technologies offer the potential for long-term solutions by addressing the genetic root causes of the condition. A surge in clinical trials and research investments aimed at finding effective treatments for dystrophic epidermolysis bullosa is propelling market growth. Collaborations between pharmaceutical companies and research institutions are driving innovation in the field.
Patient advocacy groups are playing a vital role in raising awareness about dystrophic epidermolysis bullosa and advocating for better access to care and treatments. Their efforts are fostering a more supportive environment for individuals affected by dystrophic epidermolysis bullosa. Also, regulatory agencies are streamlining the approval process for dystrophic epidermolysis bullosa therapies. This support reduces the time it takes for promising treatments to reach the market, benefiting patients with dystrophic epidermolysis bullosa. The development of advanced wound care products specifically designed for DEB patients is enhancing the quality of life for those living with the condition. These products promote better wound healing and symptom management. Moreover, collaborative efforts among international healthcare organizations and research institutions are accelerating the exchange of knowledge and expertise in the field of dystrophic epidermolysis bullosa, which is expected to accelerate market growth in the coming years.
Countries Covered:
• United States
• Germany
• France
• United Kingdom
• Italy
• Spain
• Japan
Analysis Covered Across Each Country:
• Historical, current, and future epidemiology scenario
• Historical, current, and future performance of the dystrophic epidermolysis bullosa market
• Historical, current, and future performance of various therapeutic categories in the market
• Sales of various drugs across the dystrophic epidermolysis bullosa market
• Reimbursement scenario in the market
• In-market and pipeline drugs
This report also provides a detailed analysis of the current dystrophic epidermolysis bullosa marketed drugs and late-stage pipeline drugs.
In-Market Drugs:
• Drug Overview
• Mechanism of Action
• Regulatory Status
• Clinical Trial Results
• Drug Uptake and Market Performance
Late-Stage Pipeline Drugs:
• Drug overview
• Mechanism of action
• Regulatory status
• Clinical trial results
• Drug uptake and market performance
Competitive Landscape of Key Players :
The competitive landscape of the dystrophic epidermolysis bullosa market has been studied in the report with the detailed profiles of the key players operating in the market.
Some of these Key Players:
- Fibrocell Science
- Krystal Biotech
- Abeona Therapeutics
- Phoenix Tissue Repair
Ask Analyst for Customization and Explore Full Report With TOC & List of Figures: https://www.imarcgroup.com/request?type=report&id=6576&flag=C
If you need specific information that is not currently within the scope of the report, we will provide it to you as a part of the customization.
About Us
IMARC Group is a leading market research company that offers management strategy and market research worldwide. We partner with clients in all sectors and regions to identify their highest-value opportunities, address their most critical challenges, and transform their businesses.
IMARC’s information products include major market, scientific, economic and technological developments for business leaders in pharmaceutical, industrial, and high technology organizations. Market forecasts and industry analysis for biotechnology, advanced materials, pharmaceuticals, food and beverage, travel and tourism, nanotechnology and novel processing methods are at the top of the company’s expertise.
Media Contact:
Company Name: IMARC Group
Contact Person: Elena Anderson
Email: sales@imarcgroup.com
Phone: +1-631-791-1145
Address: 134 N 4th St
City: Brooklyn
State: NY
Country: United States
Website: https://www.imarcgroup.com/