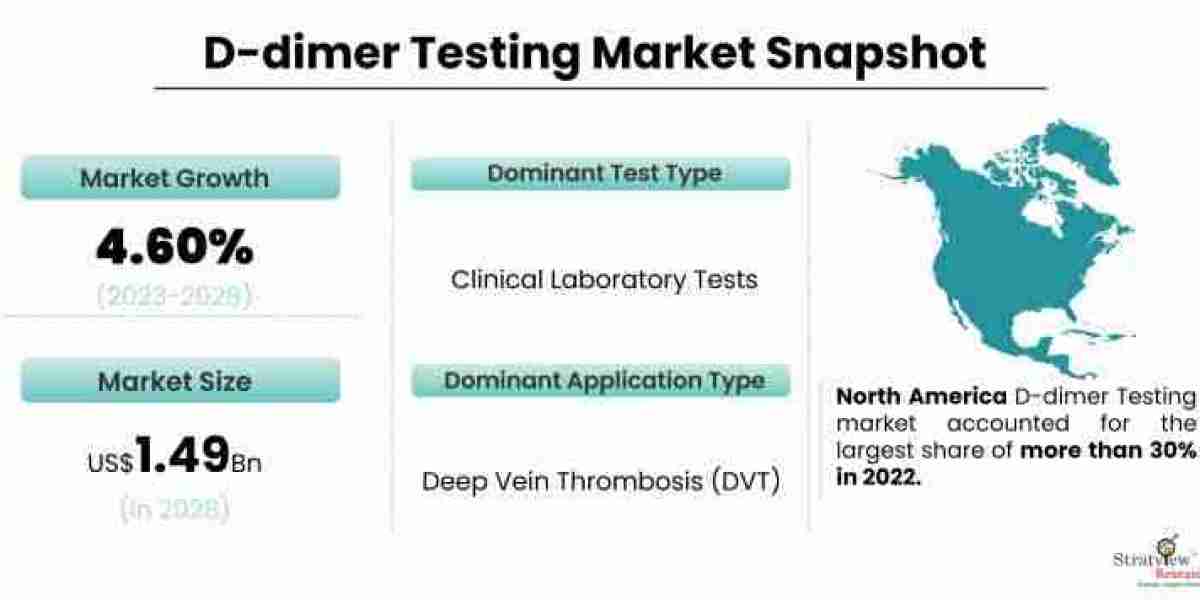
D-Dimer testing plays a crucial role in the diagnosis and management of thrombotic and fibrinolytic disorders. It is a blood test that measures the levels of D-Dimer, a protein fragment produced when a blood clot dissolves in the body. Elevated D-Dimer levels can indicate the presence of blood clots or the risk of developing a clotting disorder. The D-Dimer testing market has witnessed significant growth in recent years, driven by the rising incidence of thrombotic disorders and the increasing adoption of advanced diagnostic technologies. However, like any other market, the D-Dimer testing market also faces its fair share of challenges. In this article, we will discuss some of these challenges and potential strategies to overcome them. The D-dimer testing market is estimated to grow from USD 1.14 billion in 2022 to USD 1.49 billion by 2028 at a healthy CAGR of 4.60% during the forecast period.
Lack of Awareness: One of the primary challenges in the D-Dimer testing market is the lack of awareness among healthcare professionals and patients. Many individuals, including physicians, are not fully aware of the significance of D-Dimer testing in diagnosing thrombotic disorders. This lack of awareness can lead to underutilization of D-Dimer tests and delayed diagnosis. To overcome this challenge, it is crucial to invest in educational initiatives targeted at healthcare professionals to increase their understanding of the clinical utility of D-Dimer testing. Furthermore, raising awareness among patients about the importance of early detection and the role of D-Dimer testing can encourage more proactive participation in preventive healthcare.
Variability in Test Results: D-Dimer testing involves the use of various laboratory methods and assays, which can result in variability in test results. The lack of standardization in D-Dimer assays can pose challenges in interpreting and comparing test results across different laboratories and manufacturers. This variability can lead to inconsistencies in diagnosing and monitoring thrombotic disorders. To address this issue, there is a need for standardized protocols and guidelines for D-Dimer testing. Collaboration between regulatory authorities, professional societies, and diagnostic manufacturers is essential to establish standardized methods and ensure consistency in test results.
False Positive Results: D-Dimer tests are highly sensitive but relatively less specific. This means that they can produce false-positive results, indicating the presence of blood clots when there are none. False-positive results can lead to unnecessary follow-up tests, imaging procedures, and potential patient anxiety. To overcome this challenge, it is important to develop and implement algorithms or decision rules that combine D-Dimer testing with clinical assessment and imaging studies. These algorithms can help identify patients at low risk of thrombotic disorders, reducing the number of false-positive results and unnecessary interventions.
Cost-Effectiveness: Cost-effectiveness is a significant concern in the D-Dimer testing market. Some D-Dimer assays can be expensive, especially when performed frequently or in large volumes. The cost factor can limit the widespread adoption of D-Dimer testing, particularly in resource-constrained healthcare settings. To address this challenge, there is a need for the development of affordable D-Dimer tests without compromising on accuracy and quality. Additionally, healthcare systems should explore reimbursement strategies and policies that incentivize the appropriate use of D-Dimer testing, especially in high-risk populations.
Integration of Point-of-Care Testing: Point-of-care (POC) testing offers the advantage of providing rapid results at the patient's bedside or in remote settings. However, the integration of D-Dimer POC testing into routine clinical practice faces challenges such as device availability, regulatory compliance, and quality control. Overcoming these challenges requires close collaboration between diagnostic manufacturers, regulatory authorities, and healthcare providers to ensure the development, validation, and widespread adoption of reliable and accurate POC D-Dimer tests.
In conclusion, the D-Dimer testing market has made significant strides in improving the diagnosis and management of thrombotic disorders. However, challenges such as lack of awareness, result variability, false-positive results, cost-effectiveness, and integration of POC testing still need to be addressed. By investing in education, standardization, clinical decision algorithms, cost-effective solutions, and POC testing, the D-Dimer testing market can overcome these challenges and continue to advance in providing accurate and timely diagnostic information for patients at risk of thrombotic disorders.