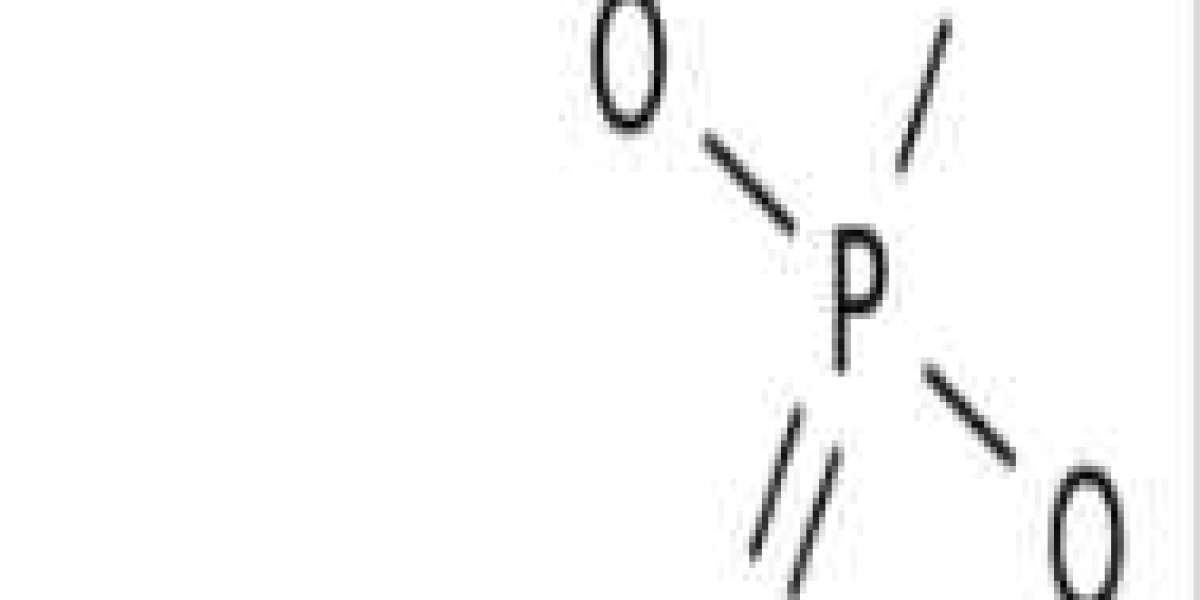
Tripotassium phosphate, also called tribasic potassium phosphate[3] is a water-soluble salt with the chemical formula K3PO4.(H2O)x (x = 0, 3, 7, 9).[4] Tripotassium phosphate is basic.
Tripotassium phosphate is produced by the neutralization of phosphoric acid.
Tripotassium phosphate has few industrial applications.
It is used as an inert, easily removed proton acceptor in organic synthesis. Some of the reactions are listed below:
The hydrate has been used to catalyze the deprotection of BOC amines. Microwave radiation is used to aid the reaction.[5]
As a catalyst for the synthesis of unsymmetrical diaryl ethers using
as the solvent. Aryl methane-sulfonates are deprotected and then followed by a nucleophilic aromatic substitution (SNAr) with activated aryl halides.[6]
As a base in the cross-coupling reaction of aryl halides with terminal alkynes. It also plays a role in the deacetonation of 4-aryl-2-methylbut-3-yn-2-ol intermediates.[7]
As the base in the cross-coupling reaction between aryl halides and phenols or aliphatic alcohols.[8]