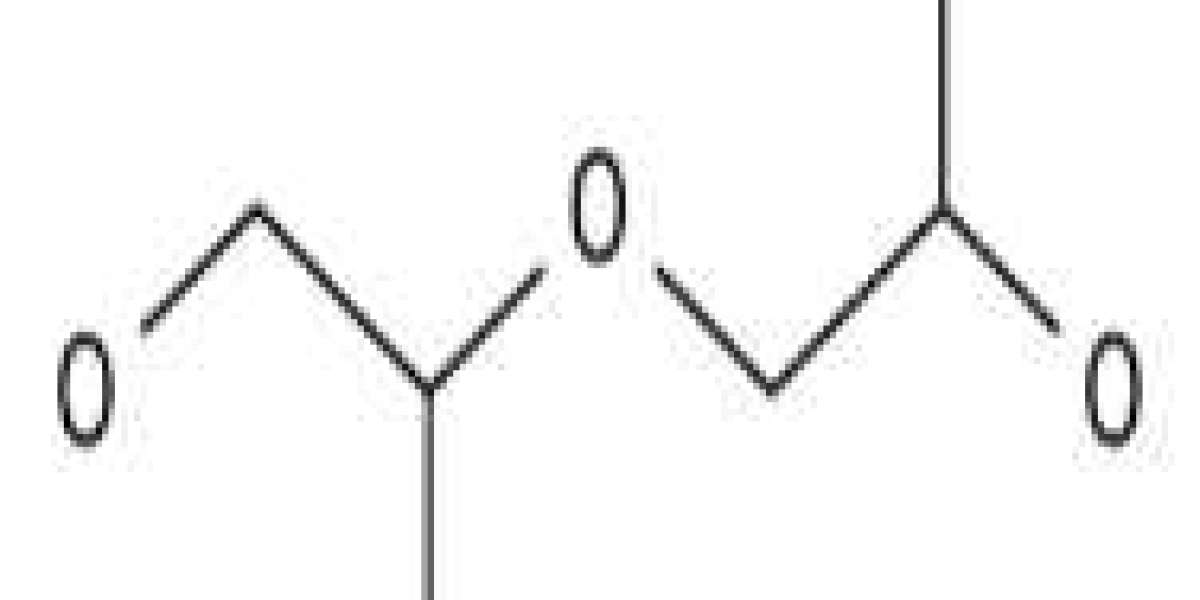
Polypropylene glycol or polypropylene oxide is a polymer (or macromolecule) of propylene glycol. [1] Chemically it is a polyether and more generally a polyalkylene glycol (PAG) HS Code 3907.2000. The term polypropylene glycol or PPG is reserved for low to moderate molar mass polymers when the nature of the end groups (usually hydroxyl groups) is still important. The term "oxide" is used for high molar mass polymers when the end groups no longer affect the polymer properties. Between 60 and 70 percent of the propylene oxide is converted into polyether polyols through a process called alkoxylation.
Polypropylene glycol is formed by ring-opening polymerization of propylene oxide. The initiator is an alcohol and the catalyst is a base, usually potassium hydroxide. When the initiator is glycol or water, the polymer is linear. Using a multifunctional initiator such as glycerol, pentaerythritol or sorbitol, the polymer branches out.
Polypropylene glycol is liquid, mostly insoluble in water, and is used to suppress foaming in industrial processes and to make polyurethane.