London
Roots Analysis has announced the addition of the “Rare Kidney Disorders Market, 2022–2035” report to its list of offerings.
More than 90 candidates targeting rare kidney disorders are currently under clinical investigation, while several novel leads are being evaluated in the early stages of development. Given the lucrative opportunity associated with these targets, this domain has gained attention of both private and public investors in the past few years. As more candidates are likely to progress towards the advanced stages of development, we expect the market to witness aggressive growth in the foreseen future.
Key Market Insights
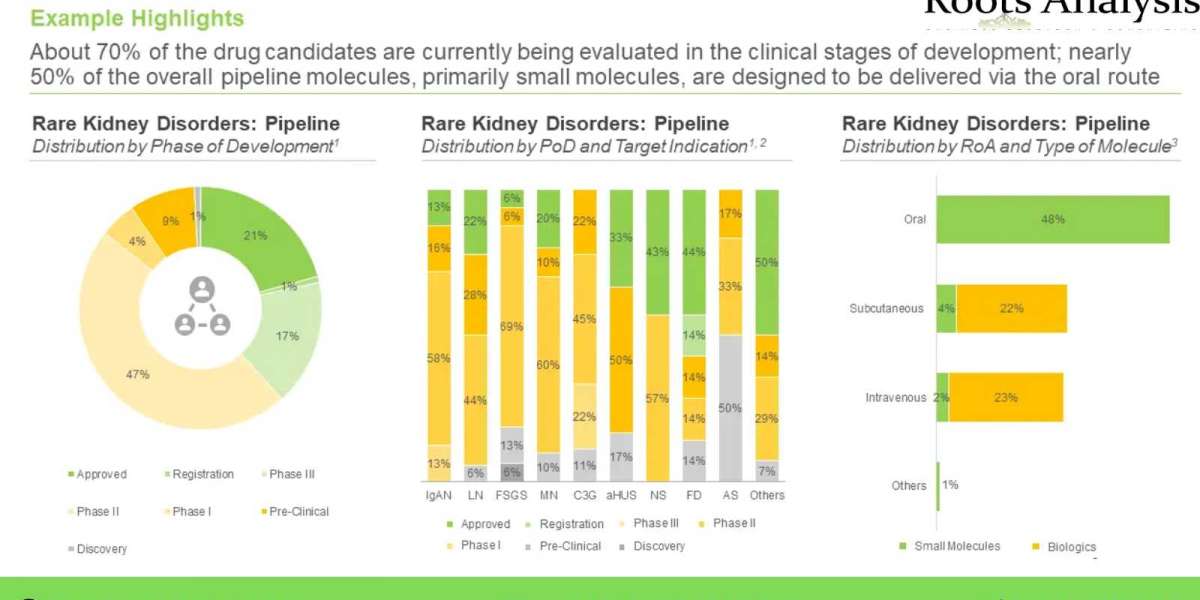
More than 125 drug candidates targeting rare kidney disorders are currently under development
Over 25 total pipeline candidates are available in the market across various geographical regions. Of these, 43% have been approved for the treatment of cystinosis, Fabry disease and lupus nephritis. Further, majority of the approved / under development drugs (47%) are designed for delivery via oral route.
Over 60 companies are engaged in the development of drugs targeting rare kidney disorders
Since 2006, 27 companies have been established in this domain. Further, around 60% of the developers are large and very large firms. In addition, majority (68%) of the players engaged in the development of drugs against rare kidney disorder are based in North America, primarily in the US.
Partnership activity in domain has increased at an annualized rate of 92%, during 2017-2021
It is worth mentioning that the maximum number of partnerships were inked in 2018 and 2019. Further, product development and commercialization agreements emerged as the most popular type of partnership model adopted by rare kidney disorder targeting drug developers, representing over 25% of the total instances.
USD 3.5+ billion has been invested by both private and public investors, since 2017
The maximum funding amount was raised through venture fundings (26%), debt financing (23%) and private equity (18%), during the period 2017-2021. Interestingly, around 85% of the funding instances were reported by players based in North America.
North America is anticipated to capture over 30% of the global market share in 2035
In the long term, close to 40% of the market revenues are expected to be generated from sales of therapeutics targeting lupus nephritis. Further, therapies designed for delivery via the oral route are expected to occupy a larger share (41%) of the overall market in 2035.
To request a sample copy / brochure of this report, please visit this
https://www.rootsanalysis.com/reports/rare-kidney-diseases-market/request-sample.html
Key Questions Answered
- Who are the leading players engaged in the development of drugs targeting rare kidney disorders?
- Which are the key drugs being developed across early and late stages of development?
- Who are the key players providing services related to kidney care?
- Which are the key indications targeted by drugs indicated for rare kidney disorders?
- What type of partnership models are commonly adopted by stakeholders engaged in this domain?
- Who are the key investors in the domain?
- Which regions have emerged as the key hubs for conducting clinical studies focused on rare kidney disorders?
- How is the current and future market opportunity likely to be distributed across key market segments?
The financial opportunity within the rare kidney disorder market has been analysed across the following segments:
- Type of Molecule
- Small Molecules
- Biologics
- Route of Administration
- Oral Delivery
- Intravenous Delivery
- Subcutaneous Delivery
- Other Delivery Routes
- Target Indications
- Alport Syndrome
- Atypical Hemolytic Uremic Syndrome
- C3 glomerulopathy
- Cystinosis
- Cystinuria
- Dense Deposit Disease
- distal Renal Tubular Acidosis
- Fabry disease
- IgA nephropathy
- Lupus Nephritis
- Refractory Gout
- Key Geographical Regions
- North America
- Europe
- Asia-Pacific
- Rest of the World
The report includes profiles of approved and clinical stage drugs indicated for rare kidney disorders (phase II / III or above). Each profile features an overview of the drug developer, clinical trial information related to the drug, clinical trial results and estimated sales revenues (if available).
- Benlysta
- Cystadrops®
- Galafold®
- Tarpeyo®
- Ultomiris®
- Thiola®
- Lupkynis™
- Sibnayal™
- Pegunigalsidase Alfa
- Saphnelo™
- Atrasentan
- Bardoxolone Methyl
- Crovalimab
- Ianalumab
- Cozaar®
- Cosentyx®
- Empaveli®
- Rituxan®
- Sparsentan
- Iptacopan
- Narsoplimab
For additional details, please visit
https://www.rootsanalysis.com/reports/rare-kidney-diseases-market.html or email sales@rootsanalysis.com
You may also be interested in the following titles:
- Cell Therapy Manufacturing Market: Industry Trends and Global Forecasts, 2022-2035
- CAR-T Cell Therapy Market (4th Edition): Industry Trends and Global Forecasts, 2022-2035
About Roots Analysis
Roots Analysis is a global leader in the pharma / biotech market research. Having worked with over 750 clients worldwide, including Fortune 500 companies, start-ups, academia, venture capitalists and strategic investors for more than a decade, we offer a highly analytical / data-driven perspective to a network of over 450,000 senior industry stakeholders looking for credible market insights.
Contact:
Ben Johnson
+1 (415) 800 3415
+44 (122) 391 1091