Roots Analysis has announced the addition of “Companion Diagnostics Development Services Market (2nd Edition), 2022-2035” report to its list of offerings.
The growing pipeline of patient-centric targeted therapies has led to a surge in demand for companion diagnostics; these tests are known to improve the success rates of late-stage trials by almost three-fold. The development and approval of these FDA classified high-risk devices requires multidisciplinary expertise and an established network of RD and production facilities that can be accessed through service providers.
Key Market Insights
Over 150 companies claim to offer companion diagnostics development services, globally
Nearly 60% of the aforementioned players are based in North America, followed by the players located in Europe (30%). Further, this segment of the industry is dominated by the presence of mid-sized players (51-200 employees), which represent 40% of the total service providers.
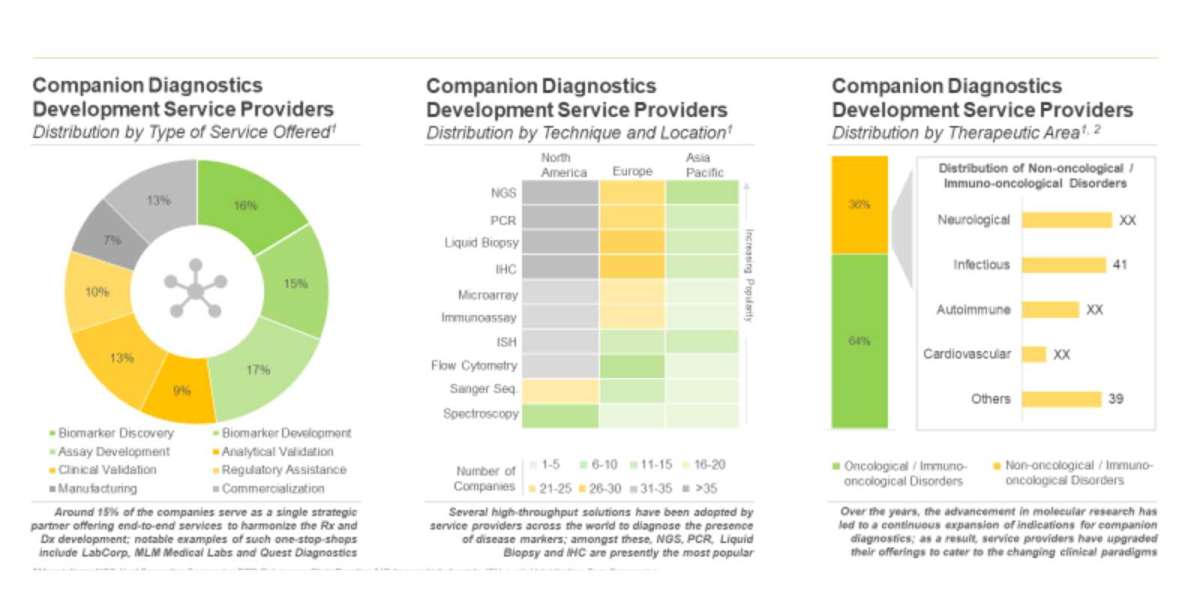
Nearly 15% players claim to act as one-stop shops, offering services, ranging from biomarker discovery and development to manufacturing and commercialization
Most of the service providers (127) provide assay development services, followed by companies (124) offering services for biomarker discovery / identification. Further, NGS is the most popular technique, employed by over 100 service providers.
Over 90 companies are actively involved in the development of companion diagnostics
Nearly 91% of the total tests can detect mutations in a single biomarker. Amongst these, ErbB gene family (HER-2 and EGFR genes), PD-L1 genes, RAS gene family (KRAS and NRAS genes), BRCA gene family (BRCA1 and BRCA2) and BRAF emerged as the key genes which are assessed by majority of the companion diagnostics.
Partnership activity in this domain has increased at a CAGR of ~25%, between 2017 and 2021
Mergers and acquisitions, product development agreements and product development and commercialization agreements account for more than 60% of the total number of deals inked during the given time period. It is worth highlighting that nearly 25% of the total number of deals were signed in 2021. Further, the maximum number (177) of partnerships have been inked for companion diagnostics being developed for the treatment of oncological disorders.
North America is expected to capture ~60% share in the companion diagnostics development services market by 2035
Owing to the high costs associated with the clinical validation of companion diagnostics, a significant proportion (~46%) of service revenues is generated from this step, in 2035. In terms of analytical technique used, service contracts involving NGS are anticipated to contribute to more than 35% of the total service revenues generated in 2035. Further, close to 90% of the total service revenues, in 2035, are generated from companion diagnostics development projects for cancer indications.
To request a sample copy / brochure of this report, please visit https://www.rootsanalysis.com/reports/297/request-sample.html
One of the key objectives of the report was to estimate the existing companion diagnostics market size and the potential future growth opportunities for companion diagnostics development service providers. Based on multiple parameters, such as the service cost of various steps involved in companion diagnostics development and manufacturing, and partnerships inked in the last few years for outsourcing of such operations, we have developed informed estimates on the evolution of the market for the time period 2022- 2035. An insightful analysis of companies segregated on the basis of their likelihood to enter into collaborations with companion diagnostics service providers. The chapter features a list of 300+ drug developers sponsoring clinical trials of therapies targeting several disease-specific biomarkers. The players have been shortlisted based on relevant parameters, namely number of biomarker-focused clinical trials sponsored and the time to market their proprietary personalized medicine products.
For additional details, please visit
https://www.rootsanalysis.com/reports/view_document/companion-diagnostics-services/297.html or email sales@rootsanalysis.com
You may also be interested in the following titles:
- Targeted protein degradation market, 2022-2035
- Cell Therapy Manufacturing Market, 2021-2030
- Single-Use Upstream Bioprocessing Technology / Equipment Market, 2022-2035
About Roots Analysis
Roots Analysis is one of the fastest growing market research companies, sharing fresh and independent perspectives in the bio-pharmaceutical industry. The in-depth research, analysis and insights are driven by an experienced leadership team which has gained many years of significant experience in this sector. If you’d like help with your growing business needs, get in touch at info@rootsanalysis.com
Contact:
Ben Johnson
+1 (415) 800 3415
+44 (122) 391 1091
Facebook - https://www.facebook.com/RootsAnalysis
LinkedIn - https://www.linkedin.com/company/roots-analysis/mycompany/
Twitter - https://twitter.com/RootsAnalysis
Medium - https://medium.com/@RootsAnalysis
Pinterest - https://in.pinterest.com/RootsanalysisPin/_saved/