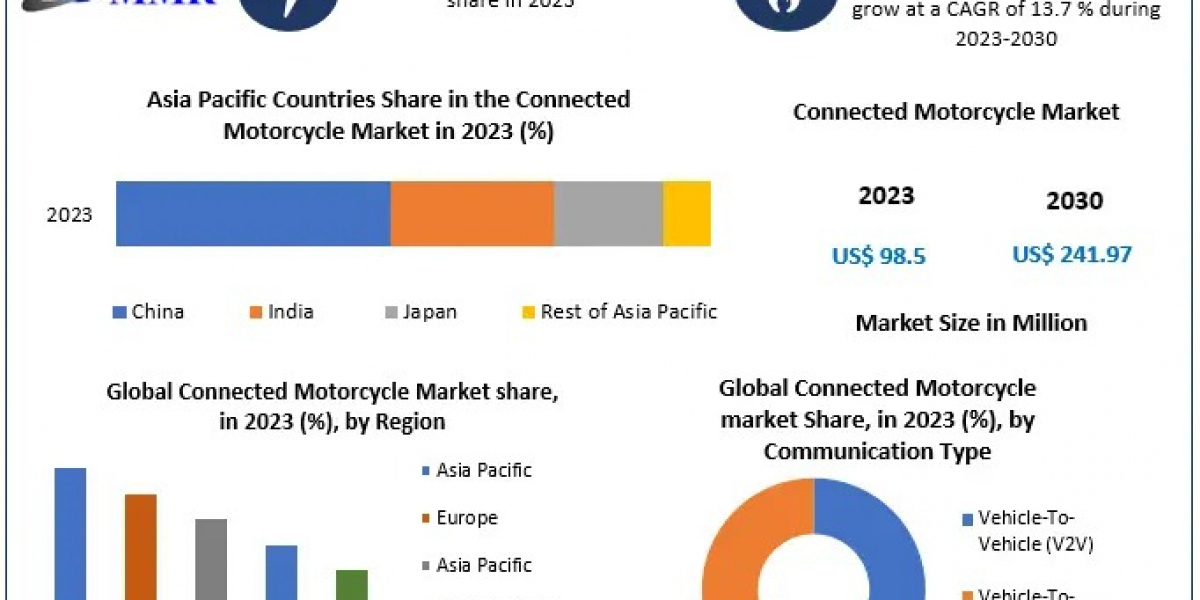
ISO 13485 certification is a vital standard for organizations involved in the design, production, installation, and servicing of medical devices. This certification ensures that companies meet stringent quality management system requirements specific to the medical device industry. In Bahrain, where the healthcare sector is rapidly evolving, attaining ISO 13485 Certification in Bahrain can significantly enhance an organization’s credibility and operational efficiency. This blog post will explore the implementation of ISO 13485 in Bahrain, the services available to support certification, and the ISO 13485 audit process in the region.
ISO 13485 Implementation in Bahrain
Implementing ISO 13485 in Bahrain involves a structured approach tailored to the specific needs of the medical device sector. The implementation process generally follows several key stages:
Gap Analysis: The first step in the implementation process is conducting a gap analysis to assess the current quality management system against ISO 13485 requirements. This analysis helps identify areas that require improvement and allows organizations to develop a tailored action plan to bridge those gaps.
Training and Awareness: Staff training is crucial to successful implementation. Employees at all levels must understand the importance of ISO 13485 and their roles in maintaining compliance. Training programs can be tailored to specific functions within the organization, ensuring that everyone understands the quality management processes and procedures.
Development of Documentation: A robust documentation system is a cornerstone of ISO 13485 compliance. Organizations must create comprehensive documentation that includes quality manuals, standard operating procedures (SOPs), and work instructions. This documentation provides clear guidance on processes and helps ensure consistent quality in the production and servicing of medical devices.
Implementation of the Quality Management System: With training and documentation in place, the organization can implement the quality management system. This involves defining processes, assigning responsibilities, and establishing metrics to monitor quality performance. The focus should be on continuous improvement, risk management, and compliance with relevant regulations.
Monitoring and Measurement: Once the quality management system is operational, continuous monitoring and measurement of processes are essential to ensure that quality objectives are met. Organizations should regularly review performance data, customer feedback, and audit results to identify areas for improvement and take corrective actions as necessary.
By following these steps, organizations in Bahrain can successfully implement ISO 13485 Implementation in South Africa and create a comprehensive quality management system that enhances their ability to produce safe and effective medical devices.
ISO 13485 Services in Bahrain
Bahrain offers various services to support organizations seeking ISO 13485 certification. These services are designed to streamline the certification process and ensure compliance with the standard.
Consulting Services: ISO 13485 consultants in Bahrain play a crucial role in guiding organizations through the certification journey. They assist in conducting gap analyses, developing documentation, and establishing quality management processes tailored to the medical device industry. These consultants bring extensive experience and expertise to help organizations navigate the complexities of ISO 13485 compliance.
Training Programs: Training services are essential to equip employees with the knowledge and skills necessary to maintain compliance with ISO 13485. Various training providers in Bahrain offer courses focused on the standard’s requirements, risk management, and internal auditing. Customized training sessions can be organized to address the specific needs of the organization, ensuring that all employees understand their responsibilities regarding quality management.
Documentation Support: Creating and maintaining the required documentation is critical for ISO 13485 compliance. Service providers in Bahrain assist organizations in developing quality manuals, SOPs, and records that meet ISO 13485 Services in Bangalore requirements. This documentation not only supports the certification process but also serves as a valuable resource for ongoing quality management efforts.
ISO 13485 Audit in Bahrain
The ISO 13485 audit process is a critical step in achieving certification and ensuring the ongoing effectiveness of the quality management system. The audit is conducted by accredited certification bodies that assess compliance with the standard.
Pre-Audit Preparation: Before the actual audit, organizations should conduct a pre-audit assessment to evaluate their readiness. This involves reviewing documentation, conducting internal audits, and addressing any identified non-conformities. This preparation helps ensure that the organization is well-prepared for the certification audit.
Stage 1 Audit: Documentation Review: The first stage of the ISO 13485 audit involves a review of the organization’s documentation. Auditors assess the quality management system's alignment with ISO 13485 requirements, including policies, objectives, and records. The Stage 1 audit aims to identify any significant issues that may affect the Stage 2 audit.
Stage 2 Audit: On-Site Assessment: The Stage 2 audit includes an on-site assessment of the organization’s operations. Auditors evaluate the implementation of the quality management system, focusing on process effectiveness, compliance with procedures, and employee engagement in quality management. This stage involves interviews with employees, observations of processes, and review of records to ensure that the system functions as intended.
Certification Decision: After completing the audit, the certification body will determine whether the organization meets ISO 13485 requirements. If successful, the organization will receive ISO 13485 certification, validating its commitment to quality management in the medical device industry. Regular surveillance audits are conducted to ensure continued compliance and identify opportunities for improvement.
Conclusion
ISO 13485 certification in Bahrain is an essential step for organizations involved in the medical device sector to demonstrate their commitment to quality management and regulatory compliance. By implementing a robust quality management system, organizations can enhance their operational efficiency, improve product safety, and build trust with customers.
With a variety of ISO 13485 services available in Bahrain, including consultancy, training, and documentation support, organizations can navigate the certification process effectively. The ISO 13485 audit process ensures that organizations maintain the highest standards of quality management, ultimately contributing to better patient outcomes and a more reliable medical device industry.
Achieving ISO 13485 Registration in Bahrain not only strengthens an organization’s position in the marketplace but also enhances its reputation as a trusted provider of high-quality medical devices in Bahrain’s dynamic healthcare landscape.