Introduction
In the intricate world of aromatic compounds, anisole stands out as a fascinating and versatile player. Understanding the chemical composition of anisole is crucial for unraveling its diverse applications across industries. This blog delves into the molecular intricacies of anisole, breaking down its chemistry to provide a comprehensive understanding of how its composition plays a pivotal role in the market context.
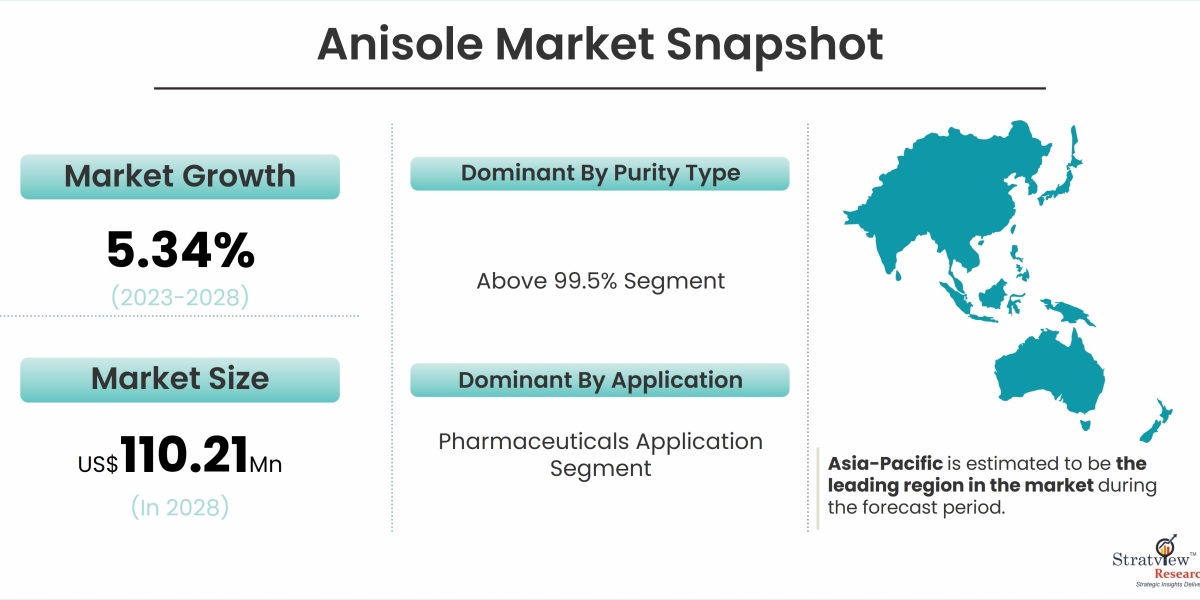
According to Stratview Research, the Global Anisole Market is expected to grow from US$ 80.62 Million in 2022 to US$ 110.21 Million by 2028 at a healthy CAGR of 5.34% during the forecast period of 2023-2028.
Anisole is an organic material that bears the scent of an anise seed. Given its distinctive physical and chemical properties, the usage of this chemical substance has been on the rise in diverse industries.
To learn more about the report, click here:
https://www.stratviewresearch.com/1783/anisole-market.html
The Molecular Symphony of Anisole
Anisole, scientifically known as methoxybenzene, belongs to the family of aromatic compounds. Its molecular structure is a testament to the elegance of organic chemistry, featuring a benzene ring—a six-carbon ring with alternating single and double bonds—substituted with a methoxy group (CH3O). This substitution imparts anisole with its characteristic sweet, anise-like fragrance, making it a prized ingredient in the fragrance and flavor industry.
Aromatics at a Glance
Benzene Ring:
The benzene ring in anisole is a planar, cyclic structure composed of six carbon atoms, each bonded to a hydrogen atom. The resonance structure of benzene results in a stable and aromatic system, where electrons are delocalized throughout the ring.
Methoxy Group:
The methoxy group consists of a single oxygen atom bonded to a methyl group (CH3). In anisole, the methoxy group replaces one of the hydrogen atoms in the benzene ring. This substitution imparts unique properties to anisole, influencing its fragrance and reactivity.
Understanding the Chemistry: Aromatic Delight
- Benzene's Aromatic Stability: The aromatic stability of benzene is a fundamental aspect of anisole's chemistry. The six π-electrons in the benzene ring form a delocalized electron cloud, creating a stable aromatic system. This stability contributes to the overall reactivity of anisole in various chemical reactions.
- Methoxy Group's Electronegativity: The electronegativity of the oxygen atom in the methoxy group influences the chemical behavior of anisole. The oxygen atom attracts electrons, creating a polar covalent bond with the adjacent carbon atom. This polarization enhances the nucleophilic nature of the methoxy group in reactions involving electrophiles.
- Aromatic Substitution Reactions: Anisole's reactivity is evident in aromatic substitution reactions, where the hydrogen atoms in the benzene ring are replaced by the methoxy group. This substitution can occur through various methods, such as Friedel-Crafts alkylation or acylation, leading to the synthesis of a variety of aromatic compounds.
Market Applications: From Chemistry to Industry
- Fragrance and Flavor Industry: A Symphony of Scents: The aromatic composition of anisole is the cornerstone of its success in the fragrance and flavor industry. The sweet, pleasant scent derived from its molecular structure makes it an invaluable ingredient in perfumes, colognes, and flavor formulations. Anisole's unique fragrance profile allows perfumers and flavors to create a diverse array of olfactory and gustatory experiences.
- Pharmaceuticals: Chemistry in Medicine: Anisole's chemistry finds practical application in the pharmaceutical industry. The methoxy group's nucleophilic nature and the aromatic stability of the benzene ring make anisole a key building block in the synthesis of pharmaceutical compounds. Medicinal chemists leverage these properties to create drug molecules with specific therapeutic effects.
- Polymers and Resins: Structural Elegance: The chemical composition of anisole contributes to its role in the production of polymers and resins. Anisole-containing monomers can undergo polymerization processes to form high-performance materials with tailored properties. The aromatic backbone enhances the structural integrity of these polymers, making them suitable for a range of industrial applications.
- Agrochemicals: Chemistry in Agriculture: Anisole's involvement in the synthesis of agrochemicals is a testament to its versatile chemistry. The compound serves as a precursor in the creation of pesticides and herbicides, contributing to crop protection. The chemistry of anisole plays a crucial role in the design and effectiveness of these agrochemical formulations.
Market Dynamics: Chemistry's Influence on Trends
- Technological Advancements: Unlocking New Chemistry: Advances in chemical synthesis and production technologies continually influence the anisole market. The development of novel synthetic methods and catalytic processes enhances the efficiency and sustainability of anisole production. These technological advancements not only impact market trends but also open doors to new applications and opportunities.
- Regulatory Landscape: Chemistry in Compliance: The chemical composition of anisole intersects with regulatory considerations in the market. Compliance with environmental regulations, safety standards, and chemical restrictions is paramount for manufacturers. Understanding the regulatory landscape is essential for navigating market dynamics and ensuring the responsible production and use of anisole.
Future Trends: Chemistry Paving the Way
- Green Chemistry Initiatives: Sustainable Chemistry, Sustainable Future: The future of the anisole market is likely to be shaped by green chemistry initiatives. The emphasis on sustainability and environmentally friendly practices is driving the exploration of greener production methods. Businesses that prioritize green chemistry principles may find themselves at the forefront of market trends.
- Biomedical Innovations: Chemistry in Healthcare: Ongoing research into the biomedical applications of anisole holds promise for innovative healthcare solutions. The unique chemistry of anisole, coupled with advancements in drug delivery systems and therapeutic formulations, could lead to breakthroughs in the pharmaceutical industry. Businesses that invest in these biomedical applications may tap into emerging markets.
- Collaborations and Cross-Industry Partnerships: Chemistry in Synergy: Cross-industry collaborations are poised to play a significant role in shaping the future of the anisole market. Partnerships between fragrance houses, pharmaceutical companies, and agrochemical manufacturers can lead to synergies that drive innovation and the exploration of new applications. Chemistry, in this context, becomes a collaborative endeavor.
Conclusion: Decoding the Chemistry, Charting the Future
In conclusion, understanding the chemical composition of anisole is the key to unlocking its potential across diverse industries. The aromatic symphony created by the benzene ring and the methoxy group defines anisole's fragrance, reactivity, and versatility. From perfumery to pharmaceuticals and beyond, anisole's chemistry influences market dynamics, trends, and future opportunities.
Businesses and stakeholders in the anisole market have the opportunity to leverage this understanding to navigate current trends and chart a course for the future. Whether through technological innovations, compliance with regulatory standards, or the exploration of new applications, the chemistry of anisole remains at the heart of its success in the market. Decoding this chemistry not only enriches our understanding of the compound but also paves the way for a fragrant and chemically fascinating future.
About Us
Stratview Research is a global market research firm, offering syndicated and custom research reports and growth consulting services. Our business intelligence and industry research reports offer clients insightful market data to aid strategic decision-making. These exclusive reports result from exclusive research methodology and are available for key industries such as chemicals, composites, advanced materials, technology, renewable energy, and more.
Stratview Research delivers custom research services across sectors. In case of any custom research requirements, please send your inquiry to sales@stratviewresearch.com or connect with our experts at +1-313-307-4176.