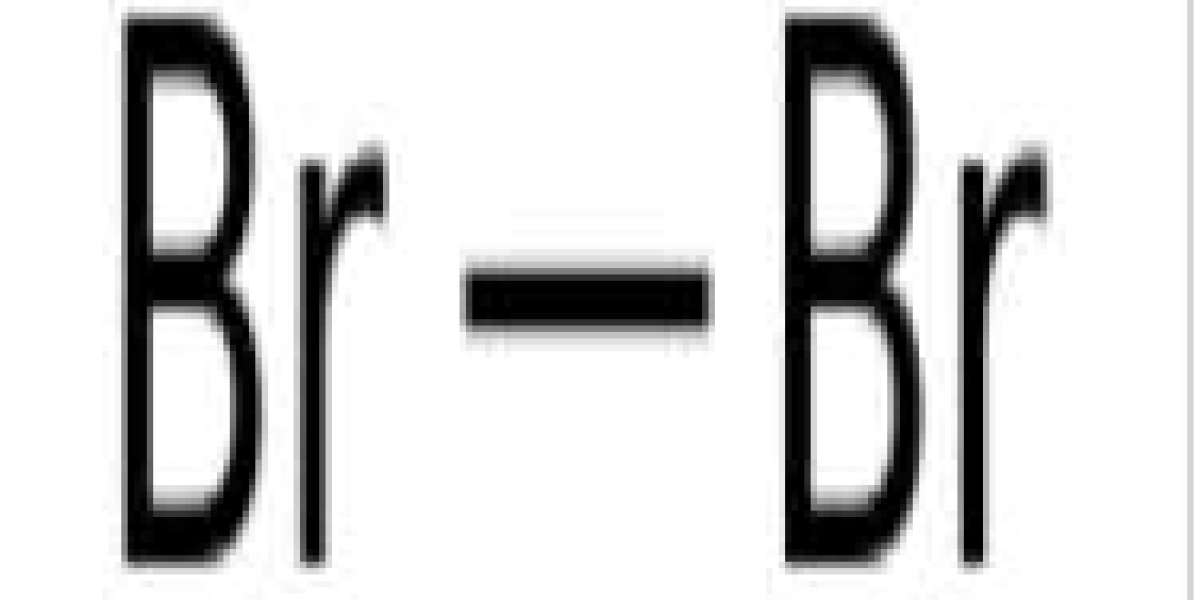Bromine is a naturally occurring element that is liquid at room temperature. It is reddish brown in color, has a bleach-like odor and is soluble in water.
Bromine is a chemical element with the symbol Br and atomic number 35. It is a volatile reddish-brown liquid at room temperature that evaporates readily to form a similarly colored vapor. Its properties are between chlorine and iodine. Independently isolated by two chemists, Carl Jacob Löwig (1825) and Antoine Jérôme Balard (1826), its name is derived from the ancient Greek βρῶμος (bromos), meaning "foul smell", referring to its pungent pungent odor.
Elemental bromine is so reactive that it does not occur in nature as a natural element, but rather as a colorless soluble crystalline mineral halide salt, similar to table salt. In fact, bromine and all halogens are so reactive that they form bonds in pairs -- not single atoms. Although it is fairly rare in the Earth's crust, the high solubility of the bromide ion (Br−) causes it to accumulate in the oceans. Commercially, the element is readily extracted from brine evaporation ponds, mainly in the United States and Israel. The mass of bromine in the ocean is about one-thirtieth that of chlorine.
Under standard conditions of temperature and pressure, it is a liquid; the only other element that is liquid under these conditions is mercury. At high temperature, organic bromine compounds are easily decomposed to generate free bromine atoms, a process that can prevent free radical chemical chain reactions. This effect makes organobromine compounds useful as flame retardants, for which more than half of the bromine produced worldwide is used annually. The same property causes ultraviolet sunlight to decompose volatile organic bromine compounds in the atmosphere, producing free bromine atoms that contribute to ozone depletion. As a result, many organobrominated compounds – such as the pesticide methyl bromide – are no longer used. Brominated compounds are still used in drilling fluids, photographic film, and as intermediates in the manufacture of organic chemicals.