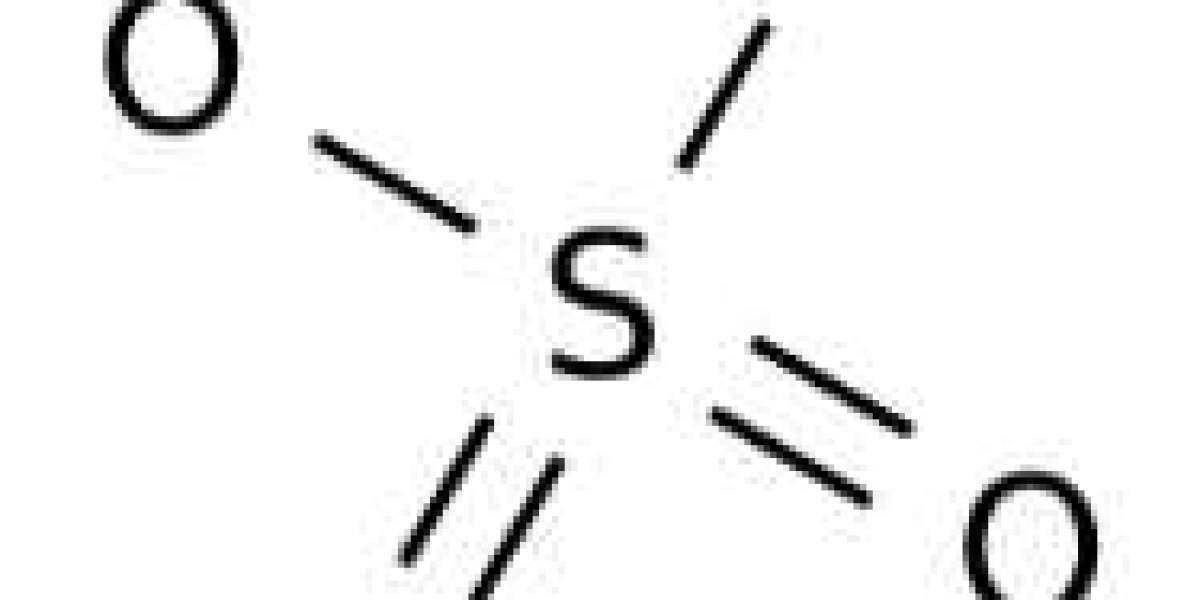
Sulfuric acid is a mineral acid consisting of one sulfur atom, four oxygen atoms, and two hydrogen atoms. The chemical formula or molecular formula for sulfuric acid is H2SO4. Sulfuric acid is one of the most important commercial chemicals. It is also known as Mattling acid or hydrogen sulfate or sulfuric acid. Sulfuric acid is a very acidic viscous liquid. It is a colorless, odorless, oily liquid that is corrosive. Sulfuric acid is a component of acid rain because it is soluble in water.
Sulfuric acid is a very acidic liquid. It is therefore used in processes such as the cleaning of metals, the extraction of impurities from petroleum, the production of chemicals such as nitric and hydrochloric acids, and the manufacture of dyes, pharmaceuticals, detergents and explosives. The molar mass of sulfuric acid is 98.079 g/mol. The density of sulfuric acid is 1.83 g/cm3. The H2SO4 molecule is covalent, with tetrahedral structure and monoclinic structure.
The chemical formula for sulfuric acid is H2SO4. According to this formula, a molecule of sulfuric acid (H2SO4) contains 2 moles of hydrogen, 1 mole of sulfur, and 4 moles of oxygen. So the molecular mass of H2SO4 is equal to 2 moles of hydrogen, 1 mole of sulfur, and 4 moles of oxygen. Since the relative atomic mass of hydrogen is 1u, that of sulfur is 32u, and that of oxygen is 16u, the molecular mass of sulfuric acid can be calculated as follows:
Sulfuric acid is a highly reactive chemical. Sulfuric acid is used in many industries such as lead-based automotive batteries, the production of various chemicals, glues and explosives, petroleum refining, metal curing, etc. Therefore, because of these wide applications, it is known as the "king of chemicals". The chemical formula for sulfuric acid is H2SO4.
Physical properties of sulfuric acid
H2SO4 is a thick, colorless, oily liquid
The density of sulfuric acid is 1.84 g/mL, the boiling point is 337℃, the melting point is 10℃.